31.10.2023
The innovative wound dressing created in Estonia shows good results in treating diabetic foot ulcers
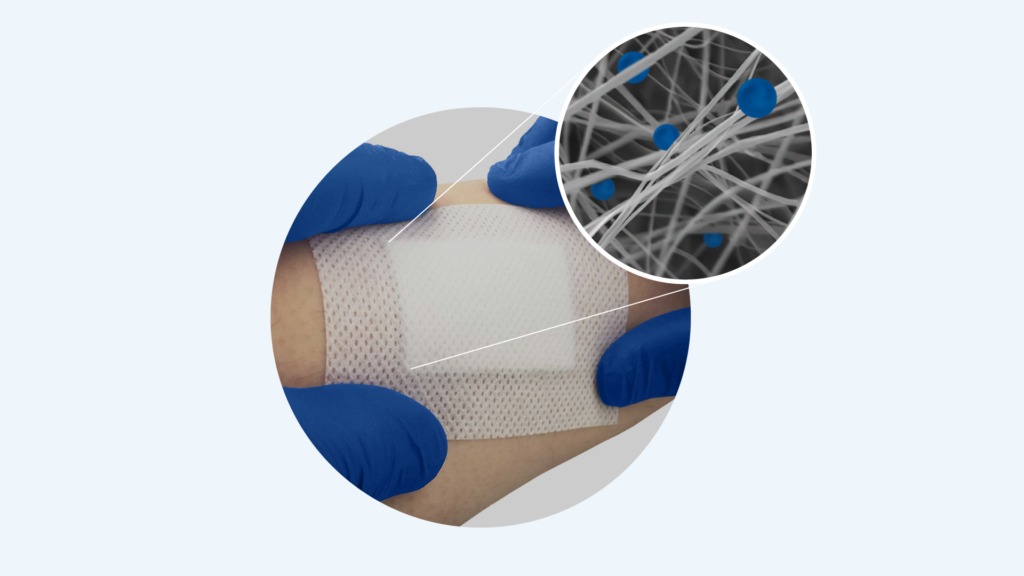
“Diabetic foot ulcers are a very serious problem around the world”, notes co-founder and CEO of Nanordica Medical, Olesja Bondarenko. This is a major reason why Nanordica Medical and North Estonia Medical Centre, members of the Tehnopol Health Tech Community, carried out research focusing on finding a more efficient treatment for foot ulcers for patients with diabetes.
“Among patients with diabetic foot ulcers, 67% are not cured within 12 weeks by the treatment currently offered, and that figure does not get any better later on. Half of those patients die within five years. That is a truly shocking statistic”, said Dr Bondarenko.
North Estonian Medical Centre tested Nanordica’s solution that would help to treat patients with diabetic foot ulcers faster, more effectively and more safely. In practical terms, this meant that Nanordica developed an innovative wound dressing for ulcers that was compared in clinical trial with the standard treatment, dressing based on ionic silver, used at the North Estonia Medical Centre.
The patients had their dressings changed during three visits, with half of them receiving the dressing from Nanordica Medical, and half using a competitor’s. During the subsequent monitoring, it was observed how the wounds healed and what side effects the different dressings caused.
There were 30 diabetic patients with foot ulcers participating in the research. “It was important for us to alleviate the suffering of patients with chronic wounds who were treated previously with various standard of care methods but without success”, said Dr Bondarenko. She gave the example of patients who had been suffering for two years already and who had tried various treatments but without any notable results.
Clinical research showed that using the Nanordica dressing helped the wound heal faster than with the dressings of competitors. “The effect was visible in most cases after already the first dressing application, as the wound shrank noticeably in size”, observed medical personnel performing the trial. These indications are very promising and an official report produced by an independent company on the results of the clinical trial should be ready in November.
There is a lot of interest in the wound dressings
Hospitals have already shown interest in using the Nanordica dressings, though the dressings have to be certified with CE marking first. A quality management system has been set up for the application for the CE mark and some of the tests have been done, though some are still ahead. The plan is to apply for the CE mark next year.
Until the CE mark is awarded, all clinical research and even each individual use of the dressing by a patient needs a separate application to the ethics committee and the permission of the Health Board. Hospitals are interested in the wound dressings and patients are contacting the company directly, so Nanordica is considering going ahead with additional clinical studies. These would aim to establish, how effective the Nanordica wound dressing is for different types of wounds. Negotiations with various hospitals have already started to this end to find the best form of cooperation for involving patients most effectively and carrying out the studies.
Thus, the Nanordica wound dressing will be most likely available to patients in the next year. “If hospitals and clinics are interested in working together with us on our research, they are always welcome to let us know”, said Dr Bondarenko.
The clinical trial was supported by the Tehnopol led Connected Health Cluster (now called Tehnopol HealthTech Community). The trial was co-funded from European Structural Fund resources.












